Abstract
Standard 200 mg/m2 high dose melphalan (HDM) yields a 500% variation in plasma melphalan exposure, i.e. area under the curve (AUC), leading to unpredictable DNA damage and widely variable clinical responses. Strategies to predict melphalan AUC have been unsuccessful given population PK models (Nath, Br J Clin Pharmacol 2010 & Cho Clin Pharmacol Ther 2017) are ineffective on an individual patient level and only explain ~20% of the variability observed, and test dose predictions have failed to accurately represent melphalan AUC for doses >100 mg/m2.
PK directed dose adjustment of HDM would require a 24-hour turnaround for sample collection, shipping, processing and data analysis. Previously we described methods for measuring melphalan PK after the Day -2 HDM dose, allowing for real-time dose adjustments (Sweiss, Br J Clin Pharmacol 2020). Here, we set out to perform the first phase 1 study of AUC-targeted HDM in ASCT (NCT04483206).
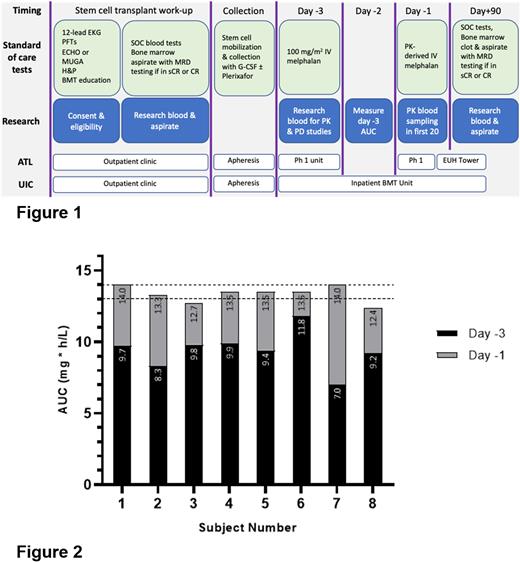
This multicenter phase 1 trial (Fig 1) is designed to test 7 AUC ranges (13-21 mg*h/L) to determine the maximum tolerated systemic exposure (MTSE). The feasibility portion focuses on the first 2 AUCs (phase A, n=20) in patients with ≥2L therapy, where we measure accuracy of achieving target AUCs of 13.5 and 14.5 mg∗h/L, respectively. Patients receive HDM 100 mg/m2 on day -3 with PK measurement and subsequent dose-adjustment on day -1 to achieve the total target AUC. 7 blood samples are drawn, processed and shipped frozen for LC-MS/MS analysis. PK parameter estimations are determined from noncompartmental analysis (NCA) and per FDA guidance used to adjust doses. Nonlinear mixed effects modeling (NONMEM) is performed in parallel to compare PK estimations. Using observed first-dose HDM clearance (Clobs) from NCA and assuming that Cl is consistent between doses, linear dose-proportional adjustments are performed to achieve the target AUC0-inf. After a planned FDA review of these cohorts, we will begin to dose escalate to determine the MTSE with up to 5 AUC levels (15.5-19.5 mg∗h/L). Bone marrow (BM) MRD status will be assessed at Day 90 as part of the Phase B primary objective. Correlatives include measuring DNA damage in circulating PBMCs post-HDM to correlate with AUC, piloting a DNA fiber combing assay on primary MM cells from patient BM samples to quantify DNA replicative stress, and assessing PROs using NCI PRO-CTCAE v.1.0.
We have enrolled 8 patients into the first AUC cohort with 110 blood samples collected. Median age was 69 (56-75) years, with 7 (87.5%) males, and 4 (50%) Blacks. The median day -3 HDM Clobs and AUC0-inf were 22.05 (16.3-38.2) L/hr and 9.53 (6.97-11.82) mg∗h/L, respectively. All shipped blood samples were received in suitable frozen condition, and in 7 (87.5%) of 8 patients, LC-MS/MS run with NCA estimation and dose reporting was done within 12 hours of receiving samples. One patient whose samples were delayed from shipping had the dose reported on day-1 resulting in 5 of 7 day-1 samples collected. Covariates of HDM PK (i.e., FFM, hematocrit) were entered into our developed PK model to further enrich our data prediction. All 8 day -1 doses were adjusted, with a resulting median total BSA-based HDM dose of 139.6 (110.93-192.6) mg/m2. After adjustment, median day -1 Clobs and AUC0-inf were 24.48 (19.3-35.8) L/hr and 3.84 (1.7-7) mg∗h/L, respectively.
For accuracy of dose adjustment, 107 (97.3%) samples were evaluable for PK parameter estimation, with 3 concentrations excluded (n=3 patients). One subject whose last 2 day -1 samples were not collected was removed from analysis as it resulted in 19% AUC extrapolation and 33% variance between day -3 and -1 Cl values. Of 7 patients, 6 (85.7%) achieved the target AUC, with less than 10% variance in Cl between doses. (Fig 2). In the one patient, it was assessed that the day -1 Cl deviated by 24% as a result of prolonged time from drug admixture to completion of infusion leading to drug degradation. Estimated melphalan Cl generated from NCA and NONMEM correlated well (r=0.99).
This is the first trial in MM implementing a novel personalized dosing approach using drug exposure as the target to define each cohort. We demonstrate feasibility of real-time PK sampling, shipping, processing, NCA PK parameter estimation, and dose adjustment of HDM. So far we report a high rate of accuracy in achieving the target AUC which we hypothesize will deliver reliable DNA damage to MM cells. Enrollment is active and mature data will be presented at the conference.
Disclosures
Patel:Exelixis: Current Employment. Quigley:Agios: Speakers Bureau; Rigel: Other: Advisory Board; Alnylam: Speakers Bureau; Servier: Speakers Bureau. Nooka:Bristol-Myers Squibb, Janssen, Takeda, Amgen, Adaptive, GlaxoSmithKline, Sanofi, Oncopeptides, Karyophram, SecureBio, and BeyondSprings: Consultancy, Honoraria. Dhodapkar:Lava Therapeutics, Sanofi, Janssen: Membership on an entity's Board of Directors or advisory committees. Kaufman:AbbVie: Other: Member of steering committee; Incyte: Other: Member of data safety monitoring committee ; AbbVie, Genentech, and Bristol Myers Squibb: Consultancy. Joseph:Janssen: Research Funding; GSK: Research Funding; BMS: Research Funding. Lonial:Celgene, Janssen, Takeda: Research Funding; Novartis, BMS, GSK, Amgen, Merck, Janssen: Honoraria; AbbVie, Bluebird, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis Pharma, and Takeda.: Consultancy. Sborov:GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Sanofi: Consultancy; BMS: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bioline: Consultancy; Amgen: Consultancy; Pfizer: Consultancy. Phelps:Alltrna: Consultancy; Wave Life Sciences: Consultancy. Hofmeister:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Genzyme: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; BlueBird Bio: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal